Nuclear reactors are used in nuclear power stations to generate electricity all over the world. They operate using fission chain reactions. These reactions are initiated, maintained, and controlled in the reactor. In general, fission is the splitting of a heavy nucleus into two or more smaller nuclei; this will be explained in more detail further on. Turbines are driven using the captured heat energy generated by fission, generating electricity.
Fission is a process that has been occurring in the universe for billions of years. We have not only used fission to produce energy for nuclear bombs, but we also use fission peacefully everyday to produce energy in nuclear power plants. Interestingly, although the first man-made nuclear reactor was produced only about fifty years ago, the Earth operated a natural fission reactor in a uranium deposit in West Africa about two billion years ago!
The main components of a nuclear reactor are fissionable fuel, moderator, shielding, control rods, and coolant. Uranium and plutonium can be used for fuel in nuclear reactors, but special isotopes need to be used. These isotopes are low in quantity versus the other isotopes found around them, which is why there is an interesting process, to make these fuels more fissionable, called breeding. Moderators help the fission chain reactions in the nuclear reactor to continue. Some of the most popular substances for moderators are graphite and (heavy) water. Shielding prevents g-radiation from escaping. Like a moderator, it slows down fast neutrons but in this case concrete can be used. One of the innovations in the CANDU reactors is that they use heavy water as a moderator and shielding. Control rods are obviously there to help control the fission process. They are made of strong neutron absorbing materials and are responsible for not only controlling the fission reaction but in helping shut down the reactor in case of an emergency. The coolant can be made of water, heavy water, and liquid metals (sodium, sodium-potassium alloy, and mercury). Not only do coolants cool the enormous quantity of heat generated by the reactor, but also they take the heat to where it can be used for power generation (turbines). While a nuclear reactor may not have too many main components, it is still an extremely complex machine.
One of the best examples of a nuclear
reactor is the CANDU power reactor, designed and engineered in Canada.
It uses heavy water as a moderator and shielding and is innovative in many
other ways, which is why it is such an efficient reactor. It even takes
itís fuel in a way thatís different from how most other reactors do. The
uranium is made into pellets (a cheap process) and put into tubes, this
way the reactor can fission more of the uranium and can even use other
substances (provided there is enough readily fissile material in them).
Fission
As mentioned, fission is the term used to describe the splitting of a heavy nucleus into two or more smaller nuclei. This is done by "smashing" the original atom with a neutron. When a neutron is captured by the nucleus of some heavy atoms the nucleus then becomes unstable and splits. Other neutrons are released when the nucleus splits. This process continues as a chain reaction and is called fission.
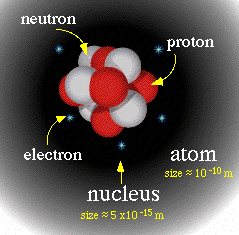
A neutron is one of the main components of an atomís core, the part of the atom that is responsible for the majority of itís mass. Just to get an idea of how the main parts of an atom are relative to each other in size protons are about 1,836 times heavier than electrons, and neutrons are about 1,838 times heavier than electrons. See Figure 1.

Nuclear reactors take advantage of the fact that the nuclei of some elements are not stable. These nuclei are radioactive, in that they emit energy and particles, collectively called "radiation." All elements have at least some isotopes that are radioactive. All isotopes of heavy elements with mass numbers greater than 206 and atomic numbers greater than 83 are radioactive.
Unstable nuclei undergo radioactive decay in several ways:
The mass number of uranium-238 declines by four and its atomic number by two when it emits an alpha particle. The number before the element name is the atomic number and that after the element name is the mass number. The totals of the atomic numbers and the mass numbers, respectively, on both sides of the nuclear reaction must be the same. This is like balancing a chemical equation; the number of atoms of each element on both sides of the reaction must be equal. The nuclei that result from radioactive decay may themselves be radioactive. Therefore, some radioactive elements have decay chains that may contain many radioactive elements; one derived from the other.
The radioactive decay of nuclei is described probabilistically. Within any given time period, a particular unstable nucleus has a fixed probability of decay. Consequently, each radioactive element is characterized by a "half-life," which is the time it takes for half the initial atoms to decay (or transmute into another element or nuclear state). At the end of one half-life, half the original element is left, while the other half is transformed into another element. After two half-lives, one fourth of the original element is left; after three half-lives, one eighth is left, and so on. This results in the build-up of decay products. If the decay products themselves decay into other elements, a whole host of radioactive materials come into being. The decay products of radioactive elements are also called daughter products or progeny.
Fission is created and maintained by free neutrons, which are unstable particles that decay naturally into a proton and electron, with a half-life of about 12 minutes.
However, it is remarkable that neutrons, when they exist together with protons in the nucleus of atoms, are stable.
Neutrons released when an atom undergoes fission are capable of causing other nuclei to undergo fission, if a moderator slows the neutrons down. Slow moving neutrons are more easily captured by the nucleus and neutrons liberated by fission travel very quickly unless moderated. Therefore, a moderator is needed. A moderator is a medium that causes neutrons to travel more slowly. Graphite, heavy water, and beryllium are all excellent moderators, capable of slowing neutrons without absorbing them because the neutrons "bounce" off the nuclei of the moderator. The effect is elastic, not absorption.
Fission occurs because of the electrostatic repulsion created by the large number of positively charged protons contained in a heavy nucleus. Two smaller nuclei have less internal electrostatic repulsion than one larger nucleus. Therefore, once the larger nucleus can overcome the strong nuclear force that holds it together, it can fission. Fission can be seen as a "tug-of-war" between the strong attractive nuclear force and the repulsive electrostatic force. In fission reactions, electrostatic repulsion wins.
Controlling the chain reaction
Control rods, made of materials such as cadmium, which absorb neutrons, are used to control the rate of a chain reaction in a nuclear reactor.
The fuel
Natural uranium ore contains about 0.7% uranium-235, which is the most readily fissionable isotope of uranium. To increase the likelihood of sustaining a chain reaction for uranium, the fissionable isotope of uranium must be increased in its relative proportion through enrichment in a breeder.
A critical mass of fissionable material is the minimum mass that will produce a nuclear explosion. To produce a sustainable nuclear chain reaction requires more material than the critical mass.

The products shown in the above equation (and Figure 4) are only one set of many possible product nuclei. Fission reactions can produce any combination of lighter nuclei so long as the number of protons and neutrons in the products sum up to those in the initial fissioning nucleus. As with fusion, a great amount of energy can be released in fission because for heavy nuclei, the summed masses of the lighter product nuclei is less than the mass of the fissioning nucleus.
Energy production
A very large amount of energy is released when an atom undergoes fission (about 200 MeV). In a typical fission reaction, the energy released is distributed as follows: 170 MeV of kinetic energy of fission fragments, 5 MeV of kinetic energy of neutrons, 15 MeV of energy beta particles and gamma rays, and 10 MeV as energy of antineutrinos.
Nuclei are tightly bound together by the strong nuclear force and each nucleus has a characteristic binding energy. This is the amount of energy it would take to completely break up a nucleus and separate all the neutrons and protons in it. Typically, binding energy increases by several megaelectron-volts (MeV) for every proton or neutron added to a nucleus. (Since protons and neutrons are constituent particles of nuclei, they are known collectively as nucleons.) The release of nuclear energy derives from the differences in binding energy between the initial nucleus (or nuclei) and relative to the end products of the nuclear reaction, such as fission or fusion.
It must be stressed that the binding energy is the amount of energy that would have to be added to the nucleus to break it up. It can be thought of (approximately) as the amount of energy liberated when a nucleon is drawn into the nucleus due to the short-range nuclear attractive force. Since energy and mass are equivalent, nuclei with higher binding energy per nucleon have a lower atomic weight per nucleon.
The key to release of nuclear energy from fission of heavy elements and fusion of light elements is that elements in the middle of the periodic table of elements, with intermediate mass numbers have a higher binding energy per nucleon (that is a lower atomic weight per nucleon). Therefore, when a heavy nucleus is fissioned, the resultant products of the nuclear reaction have a slightly smaller combined nuclear mass. This mass difference is converted to energy during nuclear fission.
Mass is not conserved in a nuclear reaction. The products formed during nuclear fission have a slightly lower mass, due to the nuclear mass defect. The conversion of a small amount of the mass of the nucleus of an atom into energy is what produces nuclear energy.
The energy released by fission can be calculated by finding the difference between the mass of the parent atom and neutron, and the masses of the daughter atoms and emitted neutrons. This is the small amount of the mass of the original nucleus of the atom that was converted into energy. In principle, all mass and energy are equivalent in a proportion defined by Albert Einstein's famous equation
which is used to convert this mass "lost" into energy.
Since the speed of light is a very large number--300 million meters per second--a small amount of mass is equivalent to a very large amount of energy. For instance, one kilogram (about 2.2 pounds) of matter is equivalent to
This is a huge of amount of energy, equivalent to the energy content of over three million metric tons of coal.
Heavy atoms such as uranium or plutonium can be split by bombarding them with neutrons. The resultant fragments, called fission products, are of intermediate atomic weight, and have a combined mass that is slightly smaller than the original nucleus. The difference appears as energy. As explained in the previous section, this mass difference arises from the binding energy characteristics of heavy elements compared to elements of intermediate atomic weight. Since the binding energy of the fission products per nucleon is higher, their total nucleonic mass is lower. The net result is that fission converts some of the mass of the heavy nucleus into energy.
The energy and mass aspects of the fission process can be explained mathematically as follows. Let the total binding energy of the heavy nucleus and the two fission products be Bh, Bf1, and Bf2, respectively. Then:
Amount of energy released per fission
Amount of mass converted to energy
This energy appears in various forms: the kinetic energy of the neutrons, the vibrational energy of the fission fragments, and gamma radiation. All of these forms of energy are converted to heat by absorption in with the surrounding media in the reactor, mainly the coolant and the moderator (for thermal reactors). The most basic fission reaction in nuclear reactors involves the splitting of the nucleus of uranium-235 when it is struck by a neutron. The uranium-235 first absorbs the neutron to yield uranium-236, and most of these U-236 nuclei split into two fission fragments. Fission reactions typically also release two to four neutrons (depending on the speed on the neutrons inducing the fission and probabilistic factors). One of these neutrons must trigger another fission for a sustained chain reaction. The fission reactions in a nuclear reactor can be written generically as follows:
The uranium-236 nucleus does not split evenly into equal fission fragments. Rather, the tendency, especially with fission induced by thermal neutrons, is for one fragment to be considerably lighter than the other. Figure 9 (not available in on-line version of report) shows the distribution of fission products due to fission with the slow neutrons and fast neutrons. It can be seen that the fission product atomic numbers are concentrated in the ranges from about 80 to 105 and from about 130 to 150 in thermal reactors. An example of a fission reaction is:
While many heavy nuclei can be fissioned with fast neutrons, only a few can be fissioned with "slow" neutrons. It turns out that, with some exceptions, like plutonium-240, only nuclei that can be fissioned with slow neutrons can be used for sustaining chain reactions. Isotopes with nuclei that can be fissioned with zero energy neutrons (in practice neutrons with low energy, or "slow neutrons") are called fissile materials. Generally, these are the odd-numbered isotopes, such as uranium-233, uranium-235, plutonium-239, and plutonium-241. Other heavy nuclei, like uranium-238, can be fissioned with fast neutrons, and so are fissionable, but not fissile.
There are only three fissile isotopes of practical importance: uranium-233, uranium-235, and plutonium-239. Of these, only uranium-235 occurs naturally in significant quantities. The other two occur in trace quantities only.
Breeding in more detail
To obtain plutonium-239 and uranium-233 in amounts useful for nuclear energy production, they must be manufactured from materials that occur in relative abundance. Plutonium-239 is produced from reactions following the absorption of a neutron by uranium-238; uranium-233 is produced by neutron absorption in thorium-232. Uranium-238 and thorium-232 are called fertile materials, and the production of fissile materials from them is called breeding. The reactions for plutonium-239 are
For uranium233 the reactions are:
The symbol Pa stands for the element protactinium.
Applications
Nuclear reactors are already in use in nuclear power plants all over the world. Although many of them operate differently with respect to fuels, moderators, and other components, the process of fission is used in all of them.
The military also uses this excellent method of power generation in nuclear submarines. Nuclear power can be used in many applications where power generation required.
Nuclear reactors generate little emissions, can run for a long time without refueling, and are efficient. The only problem is the disposal and/or recycling of used uranium and plutonium.
The Voyager space probes carried devices called radioisotope thermal generators. (RTGs) These devices are simply amazing. They work on a simple principle. With semi conductor type materials, a current can be established by heating one side while cooling the other. The effect is known as the thermoelectric effect. This is very advanced and not quite in regular use, and is discussed in the future of nuclear reactors, below.
Future
Nuclear reactors, and power generation using fission in general, has a lot of unused potential. Atomic energy provides an amazing source of concentrated power. The potential applications that have been proposed are widely varied. There is room for unlimited innovation and creativity. Imagine what it would be like to have a battery that could provide power for several decades without recharging; sounds almost like science fiction! Imagine nuclear powered cars, with almost zero-emissions and run for 10 years without refueling. The possible applications are almost endless.
The future for nuclear reactors themselves lies in fission, which is out of the scope of this writing.
Possible Improvements
Nuclear power plants convert the heat-energy from the fission process into heat and use the pressure from the steam of the heated water to turn turbines. Yet, a lot of steam still comes out of nuclear power plants. More energy can be collected from the escaping steam.
Another possible improvement is already implemented in the CANDU nuclear power plants. As mentioned earlier, the uranium is made into pellets and put into "bundles" allowing for better heat transfer and better fissioning. The CANDU reactors can fission uranium with the small amount of uranum-235 that naturally exists.
Conclusion
Nuclear reactors are an excellent source of power. With almost no emissions, high safety factor, and large power generation capacity, they are an ideal method to create electricity. There are more nuclear power plants under construction and many in operation.
Although, there are many people against nuclear power they have often been proven wrong. Once nuclear power becomes more accepted it will be a very popular source of power.
The only problem is the disposal of the used fuels. Many developments are underway and many things are possible, from better disposal methods to recycling the uranium and plutonium using breeders. Research is also underway to see how all other waste can be recycled.
Nuclear power is still being developed and has a lot of untapped potential. This form of power generation will be around for a long time, and itís growth is almost inevitable with all the possible applications it can be used with.
Books
| Author | Title | Copyright | Publisher |
| Liverhant, S. E. | Nuclear Reactor Physics | 1960 | John Wiley & Sons, Inc. |
| Hyde, M. and B. | Atoms Today and Tomorrow | 1970 | McGraw-Hill |
| Blanchard, C. H. et al | Introduction to Modern Physics | 1969 | Prentice-Hall |
Web pages
Author: Dr. Jeremy Whitlock
Title: The Canadian Nuclear FAQ
Location (URL): http://www.ncf.carleton.ca/~cz725/
Date Viewed: June 4, 1997
Author: Institute for Energy and
Environmental Research
Title: IEER Reports: Basics of Nuclear
Physics and Fission
Location (URL): http://www.ieer.org/ieer/reports/n-basics.html
Date Viewed: June 4, 1997
Author: Saskatchewan Education Curriculum
and Instruction Branch
Title: Nuclear Physics - Nuclear
Fission
Location (URL): http://www.sasked.gov.sk.ca/docs/physics/u4b3phy.html
Date Viewed: June 4, 1997
Author: Victor A. Noto et al
Title: ABC's of Nuclear Science
Location (URL): http://user88.lbl.gov/NSD_docs/abc/home.html
Date Viewed: June 4, 1997
Author: Atomic Energy of Canada Limited
(AECL)
Title: AECL's Home Page
Location (URL): http://www.aecl.ca/hom_e.htm
Date Viewed: June 4, 1997
Author: Adams Atomic Engines, Inc.
Title: Topical Index of AEI
Location (URL): http://ans.neep.wisc.edu/~ans/point_source/AEI/AEI_index.html
Date Viewed: June 4, 1997